To date, there have been more clade II mpox cases reported to the Virginia Department of Health this year than in all of 2023. This serves as a reminder for providers to continue to keep mpox in mind when evaluating patients and recommend JYNNEOS vaccine for people at risk. Please check the VDH mpox dashboard for the most up-to-date data.
Clade I mpox continues to spread in Central and Eastern Africa, with spread to other countries. On November 16, 2024, the first case of clade I mpox was reported in the U.S.
For more information, please see the Important Updates section below.
Important Updates
As of November 27, 2024, enrollment in the STOMP trial, a trial evaluating tecovirimat effectiveness, has closed. Oral tecovirimat is no longer available through the STOMP Trial. More information is in the Treatment section below.
Outbreak Updates
- Since January 2024, the Democratic Republic of the Congo (DRC) has reported more than 47,000 suspect clade I mpox cases and more than 1,000 deaths. The current outbreak is more widespread than any previous DRC outbreak and has spread to some neighboring countries. Clade I mpox historically has caused more severe illness or death compared to clade II but recent data suggest clade I mpox infections in the current outbreak may not be as severe as in previous outbreaks.
- On August 7, 2024, CDC reported that the DRC outbreak continues to grow and has resulted in clade I transmission to neighboring countries, including the Republic of Congo, Central African Republic, Burundi, Rwanda, and Uganda.
- On August 14, 2024, WHO determined that the upsurge of mpox in the DRC and a growing number of countries in Africa constitute a public health emergency of international concern (PHEIC).
- On August 15, 2024, the Public Health Agency of Sweden reported the first case of clade I MPXV infection outside of Africa. Since then, several additional countries outside of Africa have reported travel-associated clade I mpox cases.
- On November 16, 2024, CDC and the California Department of Public Health announced the first known clade I mpox case in the U.S.
On November 16, 2024, CDC and the California Department of Public Health announced the first clade I mpox case in the U.S.
- The patient had recently traveled from Eastern Africa, where there is an ongoing clade I mpox outbreak.
- On November 18, CDC issued a Health Alert Network Health Advisory .
- On December 5, CDC released a letter detailing mpox updates for clinicians.
About Clade I
Clade I mpox has historically caused more severe illness and deaths than clade II mpox, which is the cause of the 2022 global outbreak. However, recent data indicate that infections from clade I mpox in the current outbreak may not be as clinically severe as in previous outbreaks.
CDC recently assessed the risk to the U.S. posed by the clade I mpox outbreak. Currently:
- The risk to the U.S. overall population remains low.
- The risk to men who have sex with men (MSM) and people who have sex with MSM, regardless of gender (via sexual transmission) is low to moderate.
Recommendations for Clinicians
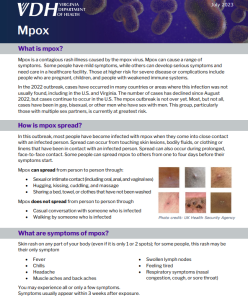
- Consider mpox as a possible diagnosis in patients with epidemiologic characteristics and lesions or other clinical signs and symptoms consistent with mpox. Ask about travel history.
- Test all suspected cases for mpox, even if the person has been vaccinated or had mpox in the past.
- Contact your local health department if a patient with suspected mpox has traveled or had contact with someone with mpox symptoms who traveled to Central or Eastern Africa the 21 days before symptom onset so that clade-specific testing can be performed through the state lab, the Division of Consolidated Laboratory Services (DCLS).
- Discuss mpox prevention and risk reduction strategies with all travelers to countries with ongoing human-to-human transmission of clade I mpox.
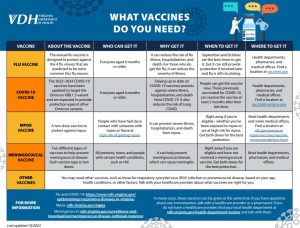
- Recommend vaccination to people who are eligible for mpox vaccine, including those who may have recent mpox exposure. Vaccines are expected to be effective for both clade I and clade II infections.
- For more information, please see CDC’s HAN Advisory.
Additional Information on the Clade I Mpox Preparedness and Response
- CDC Mpox Updates for Clinicians (12/5/24)
- CDC HAN Alert Advisory: First Case of Clade I Mpox Diagnosed in the United States (11/18/24)
- CDC Public Health Strategies for Mpox
- CDC HAN Alert Update: Prevention Strategies for Mpox, including Vaccinating People at Risk via Sexual Exposure, for U.S. Travelers Visiting Countries with Clade I Mpox Outbreaks (9/23/24)
- CDC HAN Alert Update: Mpox Caused by Human-to-Human Transmission of Monkeypox Virus in the Democratic Republic of the Congo with Spread to Neighboring Countries (8/7/24)
- CDC COCA Call: Mpox Update: Clinical Management and Outbreaks (6/27/24)
- CDC MMWR: U.S. Preparedness and Response to Increasing Clade I Mpox Cases in the Democratic Republic of the Congo — United States, 2024 (5/16/24)
General Resources
Mpox screening, prevention, and treatment should be incorporated into routine sexual health and HIV services to ensure all patients are screened for mpox, assessed for risk factors, counseled on prevention measures, and evaluated for testing and treatment, if indicated.
VDH
- VDH Mpox Preparedness Checklist for Healthcare Facilities
- VDH Mpox Information Sheet for Healthcare Providers
- VDH Integrating Mpox into Sexual Health and HIV Care
Federal
- CDC Mpox Updates for Clinicians
- CDC Clinical Overview of Mpox
- CDC Clinical Considerations for Mpox in Children and Adolescents
- CDC Clinical Considerations for Mpox in People Who are Pregnant or Breastfeeding
- CDC Clinical Considerations for Treatment and Prophylaxis of Mpox Virus Infection in Immunocompromised People
- CDC A Guide to Taking a Sexual History
- HHS Mpox and People with HIV
- VDH Mpox Trainings for Healthcare Providers
- Understanding Mpox: A World Health Issue (self-paced course, CME available)
- HHS Mpox Briefing for Providers Who Care for Pediatric Populations (9/13/24)
- CDC COCA Call: Mpox Update: Clinical Management and Outbreaks ( 6/27/24)
- CDC/IDSA Mpox Updates webinar (6/5/24)
- CDC COCA Call: Mpox Update: Stay Up to Date on Testing, Treatment, and Vaccination
- Past COCA Calls/Webinars
- CDC HAN Alert Advisory: First Case of Clade I Mpox Diagnosed in the United States (11/18/24)
- CDC HAN Alert Update: Prevention Strategies for Mpox, including Vaccinating People at Risk via Sexual Exposure, for U.S. Travelers Visiting Countries with Clade I Mpox Outbreaks (9/23/24)
- CDC HAN Alert: Mpox Caused by Human-to-Human Transmission of Monkeypox Virus in the Democratic Republic of the Congo with Spread to Neighboring Countries (8/7/24)
- CDC Clinician Outreach and Communication Activity (COCA) Now: CDC Urges Mpox Vaccination for Those Eligible Given Continued U.S. Mpox Cases (2/12/24)
- CDC HAN Advisory: Mpox Caused by Human-to-Human Transmission of Monkeypox Virus with Geographic Spread in the Democratic Republic of the Congo (12/7/23)
- Previous Health Alert Network (HAN) Health Advisories
Testing
Commercial laboratory testing for mpox is available. VDH encourages providers to use commercial laboratories, but this testing is not free. The laboratories will bill private insurance, Medicaid, or Medicare for all testing performed. Providers may find the relevant CPT code for mpox virus testing on each commercial laboratory’s website. Providers who encounter any issues while trying to order testing should contact the laboratory’s client services.
Public health testing through the Division of Consolidated Laboratory Services (DCLS) continues to be available at no cost for patients who meet clinical and epidemiologic criteria, which includes clade-specific testing to distinguish clade I and clade II MPXV. Providers should consult with their LHD about clade specific testing.
Labs Conducting Testing and Supporting Information
Treatment
Supportive care for mpox includes pain management, skin and wound care, maintenance of fluid balance, and treatment of co-occurring sexually transmitted infections or bacterial superinfections. CDC's Clinical Considerations for Pain Management of Mpox has more details.
For most individuals with intact immune systems, supportive care and pain control will be sufficient for management. In some patients, supportive care and pain control may not be adequate and treatment should be considered. Tecovirimat or TPOXX is considered as first line treatment of mpox in people who have advanced or poorly controlled HIV or are otherwise immunocompromised, as they may be at high risk for severe disease. It is important to begin tecovirimat as early as possible in such patients.
Providers seeking oral tecovirimat must access it through CDC’s expanded access investigational new drug (EA-IND) protocol. The STOMP Trial, a trial evaluating the efficacy of tecovirmat is no longer enrolling patients in either the open or random label arm.
- Interim analysis from the STOMP trial found that tecovirimat did not reduce the time to lesion resolution or have an effect on pain among adults with mild to moderate clade II mpox and a low risk of developing severe disease. There were no safety concerns associated with tecovirimat.
If patients meet protocol eligibility criteria, they can receive tecovirimat under the EA-IND protocol. Tecovirimat is available orally or intravenously for certain patients with (or who are at high risk for) protracted or life-threatening illness, including severely immunocompromised patients, people with atopic dermatitis and other conditions affecting skin integrity, children and pregnant or breastfeeding adults.
If a provider at a local health department is requesting TPOXX through the EA-IND for a patient, they should contact VDH’s Division of Pharmacy Services (DPS) for assistance with fulfilling this request.
Private providers should contact their local health department to request TPOXX through the EA-IND. The local health department will then contact DPS to coordinate medication access.
Additional treatment details, including other therapeutics (e.g., cidofovir, brincidofovir, and VIGIV) are available on CDC’s Clinical Treatment of Mpox webpage.
- VDH TPOXX Ordering Information for Virginia Providers and LHDs
- CDC Clinical Treatment Information
- CDC MMWR Interim Clinical Treatment Considerations for Severe Manifestations of Mpox — United States, February 2023
- Mpox: Caring for the Skin (American Academy of Dermatology)
- Mpox: Treating Severe Lesions (American Academy of Dermatology)
Infection Prevention & Control
Vaccine
Featured Resources
Opens pdf to download
Opens document to download
Opens in a new window
External link will open in a new window. Click link to exit Virginia Department of Health Website.