The Division of Immunization follows Advisory Committee on Immunization Practices (ACIP) recommendations for administering vaccines.

The Pink Book
The “Pink Book,” provides physicians, nurses, nurse practitioners, physician assistants, pharmacists, and others with the most comprehensive information on routinely used vaccines and the diseases they prevent.
ACIP Schedules
What is the ACIP? Which ACIP Schedule should I be following for my child?
ACIP Recommendations
What vaccines are recommended by ACIP?
Vaccine Information Statements (VIS)
Do I have the most currect updates VIS? Can I find a VIS in another language?
CDC Vaccine Schedules App
CDC Vaccine Schedules App for Clinicians and Other Immunization Providers
The Pink Book
The “Pink Book,” provides physicians, nurses, nurse practitioners, physician assistants, pharmacists, and others with the most comprehensive information on routinely used vaccines and the diseases they prevent.
General Handling & Storage
Proper vaccine storage and handling practices play a very important role in protecting individuals and communities from vaccine-preventable diseases.Vaccine quality is the shared responsibility of everyone, from the time vaccine is manufactured until it is administered.
Vaccine Management: Handling & Storage
Food and Drug Administration Package Inserts & FDA Product Approvals. Sponsored by Immunization Action Coalition.
Vaccine Storage and Handling Toolkit
This toolkit is a comprehensive guide that reflects best practices for vaccine storage and handling from Advisory Committee on Immunization Practices (ACIP) recommendations, product information from vaccine manufacturers, and scientific studies.
Standing Orders Templates for Administering Vaccines
Sponsored by Immunization Action Coalition
Vaccine Administration
General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP)
Vaccine Schedules and Related Material
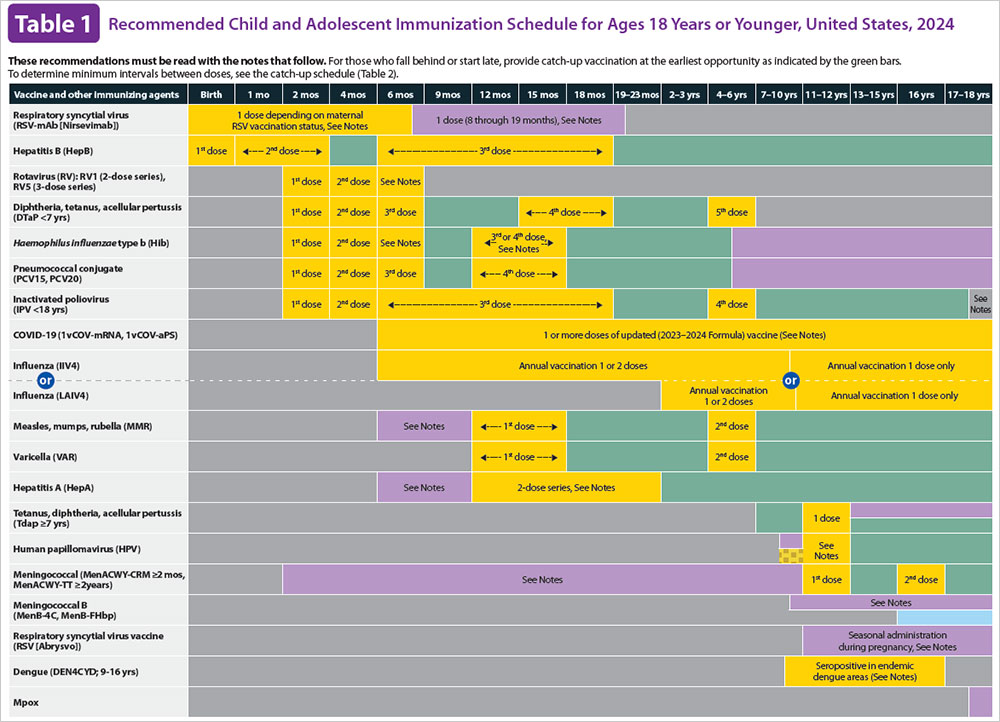
ACIP Schedules
What is the ACIP? Which ACIP Schedule should I be following for my child?
Vaccine Information Statements (VIS)
Do I have the most current updates VIS? Can I find a VIS in another language?
CDC Vaccine Schedules App
CDC Vaccine Schedules App for Clinicians and Other Immunization Providers

Vaccine Adverse Event Reporting System
Have you had a reaction following a vaccination? Contact your healthcare provider. Report an Adverse Event using the VAERS online form or the new downloadable PDF.
Vaccine S&H and other vaccine administrative information
General Handling & Storage
Proper vaccine storage and handling practices play a very important role in protecting individuals and communities from vaccine-preventable diseases. Vaccine quality is the shared responsibility of everyone, from the time vaccine is manufactured until it is administered.
FDA Package Inserts & FDA Product Approvals
Food and Drug Administration Package Inserts & FDA Product Approvals. Sponsored by Immunization Action Coalition.

Vaccine Storage and Handling Toolkit
This toolkit is a comprehensive guide that reflects best practices for vaccine storage and handling from Advisory Committee on Immunization Practices (ACIP) recommendations, product information from vaccine manufacturers, and scientific studies.

Standing Orders Templates for Administering Vaccines
Sponsored by Immunization Action Coalition
Vaccine Administration
General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP)

