January 14, 2015
Dear Colleague,
Thank you for your efforts to date to mitigate the impact of influenza on the people of Virginia. I am writing again to provide key updated information to assist you in your day to day health care decision-making. This correspondence focuses on three critical aspects of influenza for your review:
- Current influenza activity in Virginia
- Use of antiviral medications
- Reports of parotitis associated with influenza
CURRENT INFLUENZA ACTIVITY IN VIRGINIA
Influenza activity became widespread in Virginia the week ending December 13, 2014; and we expect activity to remain at high levels for several more weeks. During the week ending December 27, 2014, over 10% of the visits to Virginia emergency departments and urgent care facilities were for influenza-like illness (ILI). This amount of ILI activity is the highest that has been observed in the past three influenza seasons. Since the 2014-2015 influenza season began through January 10 2015, 104 outbreaks reported to the Virginia Department of Health (VDH) were suspected or confirmed to be caused by influenza.
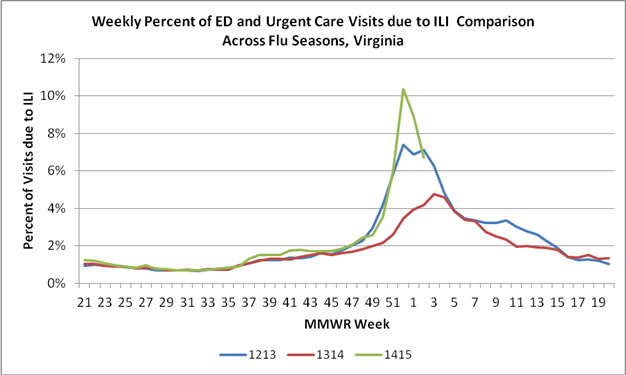
As mentioned in a prior VDH clinician letter (12/8/14) and a Centers for Disease Control and Prevention (CDC) Health Alert (http://emergency.cdc.gov/han/han00374.asp), national and state surveillance data have shown that influenza A (H3N2) viruses are the predominant strain circulating this year. Historically, H3N2-predominant flu seasons have been associated with more hospitalizations and deaths in older people and young children. Approximately two-thirds of the H3N2 viruses that have been characterized at CDC to date this season have drifted (are antigenically different) from the virus contained in this year’s flu vaccine. Despite the fact the vaccine is not a perfect match to all of the circulating flu strains, annual vaccination is still the best tool for prevention of influenza. Please continue to vaccinate your patients.
INFLUENZA AND THE USE OF ANTIVIRAL MEDICATIONS
To help protect our communities from the spread of influenza, please note the recent (1/9/2015) CDC Health Alert regarding the use of antiviral medications this flu season. (http://emergency.cdc.gov/han/han00375.asp):
- Treat any patient with suspected or confirmed influenza who is hospitalized, at high risk for influenza complications (http://www.cdc.gov/flu/about/disease/high_risk.htm), or has severe, complicated, or progressive illness as soon as possible after the development of ILI with one of three available influenza antiviral medications. These medications are reportedly underutilized and play an important role in treating influenza and reducing complications.
- Prescribe antiviral medications without waiting for confirmatory influenza testing. While antiviral drugs work best when given early (within first 48 hours of illness), therapeutic benefit has been observed even when treatment is initiated later.
- Rapid influenza diagnostic tests may not be accurate, so please remember that a negative rapid test result does not exclude a diagnosis of influenza in a patient with suspected influenza.
- Consider use of antivirals for the prevention of influenza for institutional outbreaks (such as in nursing homes or other closed populations) or for those who have contraindications to influenza vaccination.
- National antiviral supply is sufficient to meet the increased demand this season, but there may be some spot shortages for some formulations. Providers may have to contact more than one pharmacy to fill a prescription for an antiviral medication.
- Additional considerations and information on the use of antiviral medications for influenza can be found on the CDC website http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
INFLUENZA AND PAROTITIS
Since December 2014, CDC has been notified of diagnosed parotitis in persons with lab-confirmed influenza in multiple states. Parotitis is an uncommon complication of influenza. Information about persons with lab-confirmed influenza who are diagnosed with parotitis is needed to understand the occurrence of parotitis during the 2014–2015 influenza season and further explore characteristics of such cases.
If you have a patient who meets all of the following criteria, please contact your local health department (www.vdh.virginia.gov/lhd). The local health department will work with you to collect more information about this patient.
- Laboratory-confirmed influenza (e.g., rapid test, PCR, culture)
- Clinical diagnosis of parotitis or clinical signs and symptoms compatible with parotitis (i.e., “swelling of parotid gland or salivary glands”, “blurring of mandibular margin”)
- Symptom onset on or after October 1, 2014.
If a new case is identified, please collect a nasopharyngeal specimen for influenza testing and work with the local health department to receive instructions on how to test the specimen. Specimens from the affected salivary gland also would be helpful in determining etiology.
Please continue to stress other preventive health practices that may help decrease the spread of influenza and other common winter illnesses (such as norovirus), including staying home from work and school when ill, staying away from people who are sick, increasing hand washing, and using cough etiquette and respiratory hygiene practices.
Additional information about influenza, including a weekly surveillance update on influenza disease activity, is available on the VDH website at https://www.vdh.virginia.gov/epidemiology/flu.
Again, thank you for your collaborative work to prevent and control the spread of influenza in Virginia. I consider our partnership a cornerstone of the ongoing efforts to protect and promote the health of all people in Virginia.
Sincerely,
Marissa J. Levine, MD, MPH, FAAFP
State Health Commissioner

