
COVID-19 Update for Virginia
August 26, 2021
Dear Colleague:
Thank you for your continued partnership in responding to the COVID-19 pandemic. Please visit the Virginia Department of Health (VDH) website for current clinical and public health guidance, epidemiologic data, and other information. Updates on the following topics are included in this correspondence:
- FDA Grants Full Approval to Pfizer-BioNTech COVID-19 Vaccine
- Booster Dose of mRNA Vaccine for General Adult Population to Start in Mid-September
- Coadministration of COVID-19 and Influenza Vaccines
- Additional Updates about Respiratory Syncytial Virus
FDA Grants Full Approval to Pfizer-BioNTech COVID-19 Vaccine
On August 23, 2021, the U.S. Food and Drug Administration (FDA) granted full approval of the first COVID-19 vaccine, Pfizer-BioNTech COVID-19 vaccine, for persons aged 16 years and older. The vaccine will be marketed as Comirnaty. FDA’s Emergency Use Authorization (EUA) for Comirnaty will continue to cover individuals aged 12 to 15 years, and the administration of a third dose to certain immunocompromised individuals aged 12 years and older. The FDA-approved vaccine and the FDA-authorized vaccine have the same formulation and can be used interchangeably to provide the COVID-19 vaccination series without presenting any safety or effectiveness concerns. The storage, handling, and ordering process for the vaccine has not changed. FDA has updated its Comirnaty and Pfizer-BioNTech fact sheets for healthcare providers administering vaccines and for recipients and caregivers. CDC’s Advisory Committee on Immunization Practices (ACIP) will meet on August 30 to discuss updated recommendations for Comirnaty.
This approval is an important milestone that should reassure anyone concerned about getting vaccinated that the COVID-19 vaccines work and are safe. Based on results from the clinical trial, Comirnaty is 91% effective in preventing COVID-19 disease. FDA also conducted rigorous post-authorization safety surveillance regarding cases of myocarditis and pericarditis. Available short term data suggest that most individuals fully recovered, however some patients did require intensive care support. The risk was found to be highest in males aged 12 to 17 years. The myocarditis risk among vaccine recipients was also found to be much lower than the risk of myocarditis during and after actual COVID-19 disease.
With FDA’s full approval, Comirnaty can now be used in non-emergency settings. Although FDA full approval typically allows for “off-label” use of products, administration of all COVID-19 vaccines must still be done in accordance with CDC’s COVID-19 Vaccination Program requirements and the recommendations of CDC, ACIP, and FDA. This applies to both EUA and FDA approved COVID-19 vaccines. Off-label use of any COVID-19 vaccine is not recommended and could expose providers to the following risks:
- Administration of the product off-label may not be covered under the PREP Act or the PREP Act declaration; therefore, providers may not have immunity from claims.
- Individuals who receive an off-label dose may not be eligible for compensation under the Countermeasures Injury Compensation Program after a possible adverse event.
- CDC has defined the scope of the CDC COVID-19 Vaccination Program in terms of how the U.S. government-provided vaccines may be used in the program. Providers giving off-label doses would be in violation of the CDC Program provider agreement potentially impacting their ability to remain a provider in the CDC program.
- Administration fees may not be reimbursable by payers.
Moderna and Johnson & Johnson vaccines will continue to be safely administered through an EUA as the FDA reviews data about their real-world use.
Booster Dose of mRNA Vaccine for General Population to Start in Mid-September
On August 18, 2021, the U.S. Department of Health and Human Services (HHS) released a joint statement announcing the Administration’s plan for COVID-19 booster doses for the general population who have received a complete series of an mRNA vaccine. A booster dose refers to a dose of vaccine administered when the initial sufficient immune response to a primary vaccine series is likely to have waned over time. Although the COVID-19 vaccines continue to provide strong protection against severe illness, hospitalization, and death, recent evidence shows that protection against mild and moderate COVID-19 disease decreases over time in certain people. There is concern that protection against the worst COVID-19 outcomes could weaken in the months ahead, especially among people who are at higher risk for severe illness or were vaccinated during the earlier phases of the vaccine rollout. In response, VDH issued a press release and updated its Vaccination FAQs for the public and healthcare providers.
Based on this emerging data, the current proposed plan is anyone aged 18 years or older who received two doses of the Pfizer-BioNTech (Comirnaty) or Moderna vaccine should plan to get a booster dose at least eight months after they received the second dose starting the week of September 20, 2021. These recommendations (including the timeline) could change based on the FDA, ACIP, and CDC review processes. Boosters are not recommended at this time. ACIP’s meeting on August 30 will include a discussion on booster doses. Please monitor the CDC and VDH websites for the most current information as this information is subject to change; we will send an update as soon as we have more information.
At the time of the HHS announcement, a booster dose was not recommended for those who received the Johnson & Johnson vaccine because clinical trial data needed to make this decision were not available. On August 25, Johnson & Johnson reported that vaccinated individuals had strong protection persisting through eight months and that a booster dose generated a substantial increase in spike-binding antibodies in people aged 18–55 years and in those aged 65 years or older who were given a lower booster dose. These data will likely be reviewed by FDA and CDC to inform booster recommendations for this product.
In preparation for booster doses, we need to ensure enough COVID-19 vaccine providers to support the demand. If you have not already, please consider becoming a provider to support your community. For more information, please visit VDH’s Provider Enrollment Page. Additionally, the Virginia Vaccine Management and Allocation Exchange (VaxMax) supplies resources for vaccine ordering, management, and distribution for participating health care providers. If your practice was concerned before about the large quantity of minimum order size, Virginia now has a Small Shipment Redistribution Program that can provide smaller quantities to provider offices. Please consider enrolling as a COVID-19 vaccine provider today.
Coadministration of COVID-19 and Influenza Vaccines
With influenza (flu) season approaching, there may be compelling logistical advantages to offering patients COVID-19 and flu vaccines on the same day. Per CDC’s clinical considerations for COVID-19 vaccines, you can safely administer COVID-19 and flu vaccines (both live, attenuated, and non-live influenza vaccines) without regard to timing. This includes administration of COVID-19 and flu vaccines on the same day and coadministration within 14 days. When deciding whether to coadminister vaccines, please consider:
- Whether the patient is behind or at risk of becoming behind on recommended vaccines
- The patient’s risk of vaccine-preventable disease
- The reactogenicity profile of the vaccines
- The likelihood of avoiding a missed opportunity to vaccinate
Best practices for administering multiple vaccines include the following:
- Label each syringe with the name and the dosage (amount) of the vaccine, lot number, the initials of the preparer, and the exact beyond-use time, if applicable.
- Separate injection sites by 1 inch or more, if possible.
- Administer the COVID-19 vaccines and vaccines that may be more likely to cause a local reaction (i.e., adjuvanted influenza vaccines) in different limbs, if possible.
Flu vaccination will reduce the burden of flu illnesses, hospitalizations and deaths on the health care system and help conserve healthcare resources for COVID-19 and other conditions.
Additional Updates about Respiratory Syncytial Virus
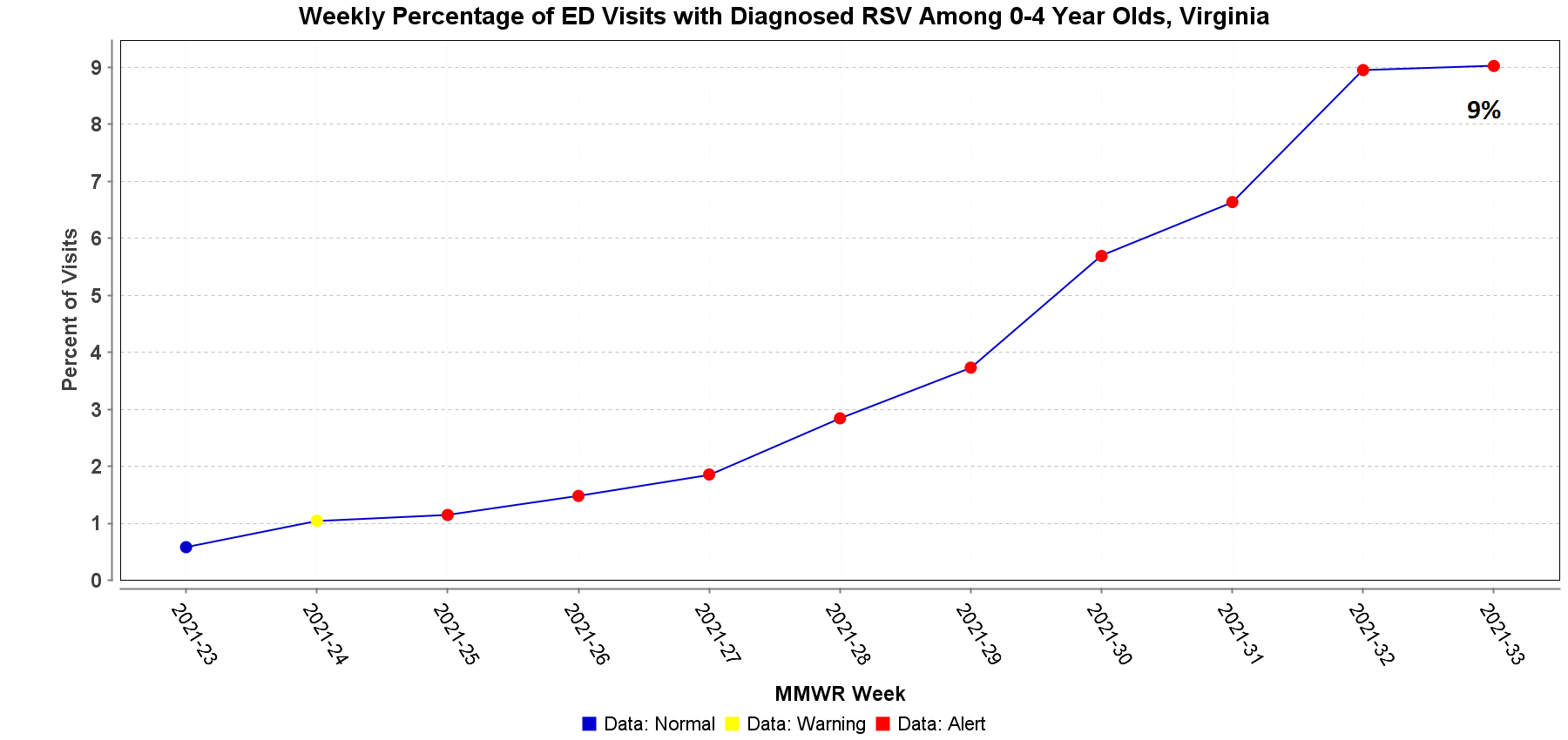
In our August 6, 2021 Dear Colleague letter, we provided information about respiratory syncytial virus (RSV) prophylaxis. Since then, the American Academy of Pediatrics (AAP) has updated its interim guidance for palivizumab prophylaxis. AAP strongly supports providers to consider using palivizumab in patients who would be candidates per current eligibility recommendations. The revised guidance updates the timing of when to administer palivizumab to account for the altered seasonality of RSV this year. The AAP recommendation applies to regions with high rates of RSV circulation, consistent with a typical fall-winter season. VDH has been seeing an increase in the number of reported RSV outbreaks. The number of RSV outbreaks reported was one in June, three in July, and eleven in August so far. During the week of August 15–21, 9% of emergency department visits among children aged 0 to 4 years in Virginia were diagnosed with RSV.
As always, I sincerely thank you for your continued partnership during these challenging times. If you have questions about COVID-19, please contact your local health department.
Sincerely,
M. Norman Oliver, MD, MA
State Health Commissioner
